Extraneal (Icodextrin) PD Solution
Indications and usage
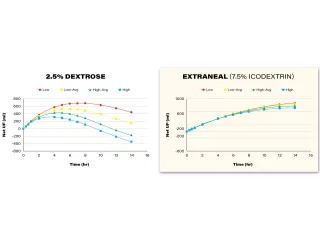
Extraneal (icodextrin) is indicated for a single daily exchange for the long (8- to 16- hour) dwell during continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD) for the management of kidney failure in patients requiring long-term kidney replacement therapy. Extraneal is also indicated to improve (compared to 4.25% dextrose) long-dwell ultrafiltration and clearance of creatinine and urea nitrogen in patients with high average or greater transport characteristics, as defined using the peritoneal equilibration test (PET).
Please see below for full Indication and Important Risk Information.
Less glucose from the start
Prescribing PD patients with icodextrin solution, such as Extraneal (7.5% icodextrin) Solution for Peritoneal Dialysis from the start, limits glucose exposure during PD therapy.1,4,6-10
Fewer treatment complications
Icodextrin solution, such as Extraneal (7.5% icodextrin) Solution for Peritoneal Dialysis, may improve clinical outcomes by increasing ultrafiltration and maintaining fluid balance.4,11-19
Less potential risk for PD dropout
When prescribed from the onset of PD, one daily bag of icodextrin solution, such as Extraneal (7.5% icodextrin) Solution for Peritoneal Dialysis for the long dwell, may increase patient time on therapy.1,19,20
What is Extraneal?
Extraneal solution is a non-glucose, 7.5% icodextrin solution for the long dwell, widely used by nephrologists for 20+ years.
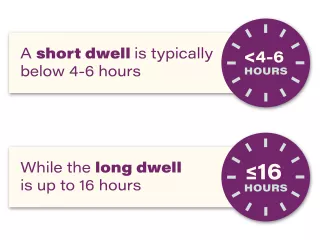
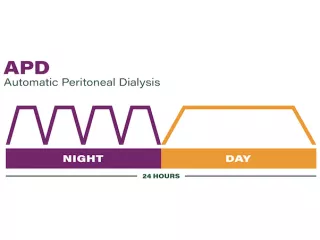
The long dwell
The dwell time is the prescribed period of time the dialysis fluid stays in the patient’s abdomen.
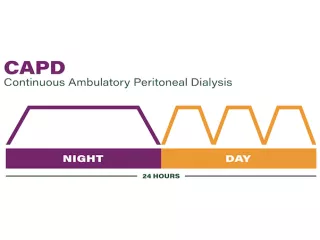
Continuous ambulatory peritoneal dialysis
Extraneal is indicated for a single daily exchange for the long dwell during continuous ambulatory peritoneal dialysis (CAPD). Exchanges are performed manually, and the long dwell is usually during the night.
Automatic peritoneal dialysis
During automated peritoneal dialysis (APD), a cycler assists the patient by automating the exchanges, the long dwell usually occurs during the day.
Learn more about the proven benefits
Want to learn more about the proven benefits when starting with Extraneal solution for peritoneal dialysis?
Did you know that ISPD has updated recommendations on the use of icodextrin?
The International Society for Peritoneal Dialysis (ISPD) guidelines recommend “high-quality goal-directed peritoneal dialysis.”21
Individualization of the prescription is a key component of this high- quality dialysis therapy, and this can be achieved through a combination of icodextrin and glucose-based solutions.21
This is contrary to the prevalent notion that only glucose solutions should be used at the beginning of PD therapy.
Get key updates related to Extraneal (icodextrin) Peritoneal Dialysis Solution
Learn more about the benefits of Extraneal and/or set up a call or meeting with an Extraneal professional.
Important Safety Information
INDICATIONS AND IMPORTANT RISK INFORMATION (IRI)
INDICATION
Extraneal (icodextrin) is indicated for a single daily exchange for the long (8- to 16- hour) dwell during continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD) for the management of kidney failure in patients requiring long-term kidney replacement therapy. Extraneal is also indicated to improve (compared to 4.25% dextrose) long-dwell ultrafiltration and clearance of creatinine and urea nitrogen in patients with high average or greater transport characteristics, as defined using the peritoneal equilibration test (PET).
IMPORTANT RISK INFORMATION
- Extraneal is contraindicated in patients with a known hypersensitivity to icodextrin, in patients with maltose or isomaltose intolerance, in patients with glycogen storage disease, and in patients with severe lactic acidosis.
- When measuring blood glucose levels in patients using Extraneal, do not use blood glucose monitoring devices using glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ)-, glucose-dye-oxidoreductase (GDO)-, and some glucose dehydrogenase flavin-adenine dinucleotide (GDH-FAD)-based methods because these systems may result in falsely elevated glucose readings (due to the presence of maltose). Falsely elevated glucose readings have led patients or health care providers to withhold treatment of hypoglycemia or to administer insulin inappropriately leading to unrecognized hypoglycemia. Falsely elevated glucose levels may be measured up to two weeks following cessation of Extraneal therapy when GDH-PQQ-, GDO-, and GDH-FAD-based blood glucose monitors and test strips are used. Additionally, other glucose-measuring technologies, such as continuous glucose monitoring systems, may or may not be compatible with Extraneal. Always contact the device manufacturer for current information regarding compatibility and intended use of the device in the dialysis patient population.
- Extraneal is intended for intraperitoneal administration only. Not for intravenous or intra-arterial administration. Aseptic technique should be used throughout the peritoneal dialysis procedure.
- Encapsulating peritoneal sclerosis (EPS), sometimes fatal, is a complication of peritoneal dialysis therapy and has been reported in patients using Extraneal.
- Serious hypersensitivity reactions to Extraneal have been reported such as toxic epidermal necrolysis, angioedema, serum sickness, erythema multiforme and vasculitis. Anaphylactic or anaphylactoid reactions may occur. If a serious reaction is suspected, discontinue Extraneal immediately and institute appropriate therapeutic countermeasures.
- Over-infusion of peritoneal dialysis solution volume into the peritoneal cavity may be characterized by abdominal distention, feeling of fullness and/or shortness of breath. Drain the peritoneal dialysis solution from the peritoneal cavity to treat over-infusion.
- Patients with insulin-dependent diabetes may require modification of insulin dosage following initiation of treatment with Extraneal. Monitor blood glucose and adjust insulin, if needed.
- Peritoneal dialysis may affect a patient’s protein, water-soluble vitamin, potassium, sodium, chloride, bicarbonate, and magnesium levels and volume status. Monitor electrolytes and blood chemistry periodically. Monitor fluid status to avoid hyper- or hypovolemia and potentially severe consequences, including congestive heart failure, volume depletion, and hypovolemic shock. Abnormalities in any of these parameters should be treated promptly under the care of a physician.
- In clinical trials, the most frequently reported adverse events occurring in ≥10% of patients and more common in Extraneal PD solution patients than in control patients, were peritonitis, upper respiratory infection, hypertension, and rash. The most common treatment‐related adverse reaction for Extraneal PD solution patients was skin rash.
Click here full Prescribing Information for Extraneal PD Solution.
Rx Only. For safe and proper use of the products mentioned herein, please refer to the appropriate Operator’s Manual or Instructions for Use.
Vantive and Extraneal are trademarks of Vantive Health LLC or its affiliates.
References
-
Takatori Y, Akagi S, Sugiyama H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1337-1344.
-
Woodrow G, Fan SL, Reid C, Denning J, Pyrah AN. Renal Association Clinical Practice Guideline on peritoneal dialysis in adults and children. BMC Nephrol. 2017;18(1):333.
-
Morelle J, Sow A, Fustin CA, et al. Mechanisms of crystalloid versus colloid osmosis across the peritoneal membrane. J Am Soc Nephrol. 2018;29(7):1875-1886.
-
Paniagua R, Ventura MD, Avila-Díaz M, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009;29(4):422-432.
-
Mujais S, Vonesh E. Profiling of peritoneal ultrafiltration. Kidney Int Suppl. 2002;(81):S17-S22.
-
Davies SJ, Brown EA, Frandsen NE, et al. Longitudinal membrane function in functionally anuric patients treated with APD: data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int. 2005;67(4):1609-1615.
-
Wang IK, Lin CL, Chen HC, et al. Risk of new-onset diabetes in end-stage renal disease patients undergoing dialysis: analysis from registry data of Taiwan. Nephrol Dial Transplant. 2018;33(4):670-675.
-
Furuya R, Odamaki M, Kumagai H, Hishida A. Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant. 2006;21(2):494-498.
-
de Moraes TP, Andreoli MC, Canziani ME, et al. Icodextrin reduces insulin resistance in non-diabetic patients undergoing automated peritoneal dialysis: results of a randomized controlled trial (STARCH). Nephrol Dial Transplant. 2015;30(11):1905-1911.
-
Gürsu EM, et al. The effect of icodextrin and glucose-containing solutions on insulin resistance in CAPD patients. Clin Nephrol. 2006;66(4):263-268.
-
Goossen K, Becker M, Marshall MR, et al. Icodextrin versus glucose solutions for the once-daily long dwell in peritoneal dialysis: an enriched systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2020;75(6):830-846.
-
Davies SJ, Woodrow G, Donovan K, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003;14(9):2338-2344.
-
Konings CJ, Kooman JP, Schonck M, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003;63(4):1556-1563.
-
Cho Y, Johnson DW, Badve S, Craig JC, Strippoli GF, Wiggins KJ. Impact of icodextrin on clinical outcomes in peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2013;28(7):1899-1907.
-
Chow KM, Szeto CC, Kwan BC, et al. Randomized controlled study of icodextrin on the treatment of peritoneal dialysis patients during acute peritonitis. Nephrol Dial Transplant. 2014;29(7):1438-1443.
-
Kim YL, Biesen WV. Fluid overload in peritoneal dialysis patients. Semin Nephrol. 2017;37(1):43-53.
-
Ho-dac-Pannekeet MM, Schouten N, Langendijk MJ, et al. Peritoneal transport characteristics with glucose polymer based dialysate. Kidney Int. 1996;50(3):979-986.
-
Morelle J, Stachowska-Pietka J, Öberg C, et al. ISPD recommendations for the evaluation of peritoneal membrane dysfunction in adults: classification, measurement, interpretation and rationale for intervention. Perit Dial Int. 2021;41(4):352-372.
-
Woodrow G, Fan SL, Reid C, Denning J, Pyrah AN. Renal Association Clinical Practice Guideline on peritoneal dialysis in adults and children. BMC Nephrol. 2017;18(1):333.
-
Johnson DW, Vincent K, Blizzard S, Rumpsfeld M, Just P. Cost savings from peritoneal dialysis therapy time extension using icodextrin. Adv Perit Dial. 2003;19:81-85.
-
Brown EA, Blake PG, Boudville N, et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40(3):244-253.