Supporting Kidney Function With CRRT
New study suggests increased CRRT utilization to better AKI survival rates in the ICU
Dr Jorge Echeverri, Medical Director, Acute Therapies at Vantive and co-author of this new study, discusses findings linking greater CRRT use to survival - and why it’s a call to action for ICU teams delivering RRT.48
Acute kidney injury: each patient is different
Acute kidney injury (AKI) is defined as an abrupt decrease in kidney function that occurs over a period of 7 days or less, encompassing both direct injury to the kidney as well as acute impairment of function.8
AKI is a heterogeneous syndrome8,9 associated with poor patient outcomes, with each patient having a unique risk profile and trajectory of disease progression.10,11
Reported incidences vary
The reported incidence of AKI among ICU patients varies from 0.5% to 78.7%12-14; up to ~25% of these patients may require renal replacement therapy (RRT).15-18
Risk of mortality
AKI is associated with an increased risk of morbidity19-29 and short- and long-term mortality.30-35
Progression to CKD
AKI is associated with an increased risk of progression to chronic kidney disease (CKD), including end-stage renal disease (ESRD).31,34,36
Hemodynamic status
Many clinicians prefer CRRT over IRRT for patients with AKI who are hemodynamically unstable.6,8
Severity of fluid overload
Clinical guidelines recommend CRRT in patients with increased intracranial pressure or generalized brain edema caused by fluid overload.8
We suggest using CRRT, rather than standard intermittent RRT, for hemodynamically unstable patients. (Grade 2B)8
KDIGO Clinical Practice Guideline for Acute Kidney Injury
CRRT enables more precise fluid management through slow, continuous fluid removal and flexible adjustment of removal intensity, compared to intermittent RRT modalities
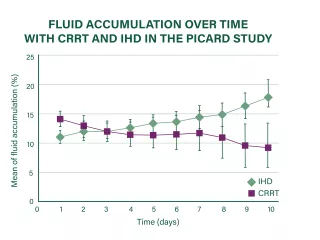
CRRT for more precise fluid management
CRRT has been shown to continuously reduce fluid accumulation in an effective and timely manner,37,38 providing the flexibility to adjust fluid removal intensity at any time according to changes in the patient’s clinical condition.8
Limited evidence suggests that CRRT may be able to provide better control of fluid management than other RRT modalities.8,38-40
Figure adapted from Bouchard J, et al. Kidney Int. 2009;76(4):455-427. Details of data collection and statistical analysis were not reported.
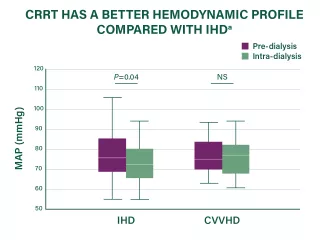
CRRT as the preferred RRT modality by many clinicians for patients with AKI who are hemodynamically unstable
Hemodynamic instability is common among critically ill patients with AKl receiving RRT (~36-70% of patients)40,41 and may be associated with an increased risk of mortality and AKI progression. 42,43
Existing medical evidence suggests that CRRT may be better able to maintain hemodynamic stability while removing fluid compared with intermittent hemodialysis (IHD) and sustained low-efficiency dialysis (SLED).44-46
aRandomized controlled trial of 80 critically ill adult patients with acute renal failure requiring dialysis in the ICUs at an institution in the US (1995-1999). Data are for IHD and CVVHD therapy during the initial dialysis day. Shown are median values with interquartile range (box borders) and extreme values (whiskers).38
CRRT may be a cost-effective therapy that may provide additional clinical and operational benefits for patients and hospitals.
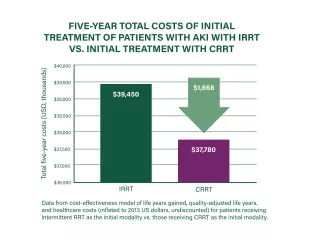
Cost effectiveness of CRRT
Initial treatment of patients with AKI using CRRT may be more cost-effective at 5 years post-RRT initiation than other RRT modalities.3
Cost-effectiveness analyses suggest CRRT to be cost-effective compared with intermittent RRT, with dialysis-dependence rate as the major driver of cost-effectiveness.47
Benefit of single-system integrated support
Critically ill patients often require extracorporeal support, with renal replacement therapy including CRRT, commonly used in the ICU.37
A review by Ronco et al. describes how modern renal replacement therapy (RRT) machines have evolved into platform-based system designs that support the independent configuration of individual extracorporeal therapies. This can help reduce the need for multiple standalone devices and simplify equipment requirements in complex care environments.37
Episode 1: Deciding when and who should start acute dialysis: From evidence to bedside practice
Hosted by Ravi Mehta, UC San Diego, featuring Marlies Ostermann, Guy’s and St. Thomas’ Foundation Trust
Episode 3: How to develop an acute dialysis care quality program: Quality metrics and continuous improvement
Hosted by Ravi Mehta, UC San Diego, featuring Theresa Mottes, Baylor College of Medicine
Important Safety Information
The Prismaflex and PrisMax Systems are intended for:
Continuous Renal Replacement Therapy (CRRT) for patients weighing 20 kilograms or more with acute renal failure and/or fluid overload.
Therapeutic Plasma Exchange (TPE) therapy for patients weighing 20 kilograms or more with diseases where fluid removal of plasma components is indicated.
Rx Only. For safe and proper use of the products mentioned herein, please refer to the appropriate Operator’s Manual or Instructions for Use.
Vantive, Prismaflex and PrisMax are trademarks of Vantive Health LLC or its affiliates.
References
-
Koyner JL, Mackey RH, Echeverri J, et al. Dialysis dependence at 90 days post discharge for patients treated with continuous renal replacement therapy (CRRT) vs. intermittent hemodialysis (IHD). Poster presented at: 28th International Conference on Advances in Critical Care Nephrology – AKI & CRRT 2023; March 29 - April 1, 2023; San Diego, California, USA.
-
Naorungroj T, Neto AS, Wang A, Gallagher M, Bellomo R. Renal outcomes according to renal replacement therapy modality and treatment protocol in the ATN and RENAL trials. Crit Care. 2022;26:269.
-
Ethgen O, Schneider AG, Bagshaw SM, Bellomo R, Kellum JA. Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients. Nephrol Dial Transplant. 2015;30(1):54-61.
-
Wald R, Gaudry S, da Costa BR, et al. Initiation of continuous renal replacement therapy versus intermittent hemodialysis in critically ill patients with severe acute kidney injury: a secondary analysis of STARRT-AKI trial. Intensive Care Med. 2023;49(11):1305-1316.
-
Ethgen O, Murugan R, Echeverri J, Blackowicz M, Harenski K, Ostermann M. Economic analysis of renal replacement therapy modality in acute kidney injury patients with fluid overload. Crit Care Explor. 2023;5(6):e0921.
-
Ostermann M, Joannidis M, Pani A, et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42(3):224-237.
-
Acute Dialysis Quality Initiative (ADQI) 17 Workgroup. ADQI 17 Figures. 2016. Accessed July 8, 2024. https://pittccmblob.blob.core.windows.net/adqi/17fig.pdf
-
KDIGO Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):1-138.
-
Aglae C, Muller L, Reboul P, et al. Heterogeneity of cause, care, and prognosis in severe acute kidney injury in hospitalized patients: a prospective observational study. Can J Kidney Health Dis. 2019;6.
-
Cerdá J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):881-886.
-
Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784-791.
-
Chawla L, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241-257.
-
Mehta S, Chauhan K, Patel A, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):91.
-
Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
-
Melo FAF, Macedo E, Fonseca Bezerra AC, et al. A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PLoS One. 2020;15(1):e0226325.
-
Jiang L, Zhu Y, Luo X, et al. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol. 2019;20:468.
-
Bouchard J, Acharya A, Cerda J, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10(8):1324-1331.
-
Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
-
De Corte W, Dhondt A, Vanholder R, et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20:256.
-
Garzotto F, Ostermann M, Martín-Langerwerf D, et al. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in critically ill patients. Crit Care. 2016; 20:196.
-
Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care. 2014;18(5):492.
-
Go AS, Hsu C, Yang JT, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol. 2018;13(6):833-841.
-
Wu V, Wu C, Huang T, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595-605.
-
Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4):e000933.
-
Brown JR, Parikh C, Ross C, et al. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg. 2014;97(1)111-117.
-
Brown JR, Hisey W, Marshall E, et al. Acute kidney injury severity and long-term readmission and mortality after cardiac surgery. Ann Thorac Surg. 2016;102(5):1482-1489.
-
Lai TS, Wang CY, Pan SC, et al. Risk of developing severe sepsis after acute kidney injury: a population-based cohort study. Crit Care. 2013;17:R231.
-
Horkan CM, Purtle SW, Mendu ML, Moromizato T, Gibbons FK, Christopher KB. The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit Care Med. 2015;43(2):354-364.
-
Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am. J. Kidney Dis. 2015;65(2)275-282.
-
Sawhney S, Marks A, Fluck N, et al. Acute kidney injury as an independent risk factor for unplanned 90-day hospital readmissions. BMC Nephrol. 2017;18:9.
-
Silver SA, Harel Z, McArthur E, et al. 30-day readmissions after an acute kidney injury hospitalization. Am J Med. 2017;130(2)163-172.
-
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009; 53(6):961-973.
-
See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160-172.
-
Srisawat N, Sileanu FE, Murugan R, et al. Variation in risk and mortality of acute kidney injury in critically ill patients: a multicenter study. Am J Nephrol. 2015;41(1):81-88.
-
Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411-1423.
-
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448.
-
Ronco C, Ricci Z, De Backer D, et al. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care. 2015;19(1):146.
-
Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the ADQI (Acute Disease Quality Initiative) group. J Am Heart Assoc. 2018;7(11):e008834.
-
Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44(6):1000-1007.
-
Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422-427.
-
Clark WR, Ding X, Qiu H, et al. Renal replacement therapy practices for patients with acute kidney injury in China. PLoS One. 2017;12(7):e0178509.
-
STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(5):502.
-
Izawa J, Kitamura T, Iwami T, et al. Early-phase cumulative hypotension duration and severe-stage progression in oliguric acute kidney injury with and without sepsis: an observational study. Crit Care. 2016;20:405.
-
Haase-Fielitz A, Haase M, Bellomo R, et al. Perioperative hemodynamic instability and fluid overload are associated with increasing acute kidney injury severity and worse outcome after cardiac surgery. Blood Purif. 2017;43(4):298-308.
-
Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36(2):610-617.
-
Fieghen HE, Friedrich JO, Burns KE, et al. The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11:32.
-
Singh A, Hussain S, Kher V, Palmer AJ, Jose M, Antony B. A systematic review of cost-effectiveness analyses of continuous versus intermittent renal replacement therapy in acute kidney injury. Expert Rev Pharmacoecon Outcomes Res. 2022;22(1):27-35.
-
Neyra JA, Echeverri J, Bronson-Lowe D, Plopper C, Harenski K, Murugan R. Association of hospital-level continuous kidney replacement therapy use and mortality in critically ill patients with acute kidney injury. Intensive Care Med. 2025 Jul;51(7):1271-1281.