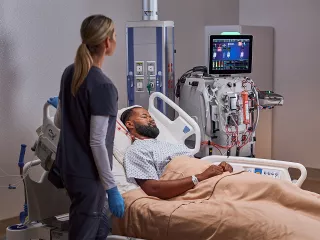
Filtersets for Individualized Acute Therapy
Vantive offers a range of innovative filtersets to meet the unique needs of critically ill patients.

CRRT — AN69 Filtersets
Biocompatible AN69 CRRT filtersets, with a unique adsorptive clearance mechanism, can be used with all CRRT modalities and can support modality change during treatment.
Therapeutic Plasma Exchange (Filtration TPE)
The TPE filterset enables removal of plasma containing abnormal large molecular weight substances and simultaneous infusion of a replacement solution.

Molecular Adsorbent Recirculating System (MARS)
The MARS filterset enables the removal of harmful drugs and poisons in combination with CRRT.
Important Safety Information
INTENDED USE
The Prismaflex ST set is a single-use device that provides blood purification through a semipermeable membrane. The Prismaflex ST set is for use only in conjunction with the Prismaflex control unit or with the PrisMax control unit (in countries where PrisMax is cleared or registered). All treatments administered via the Prismaflex ST sets must be prescribed by a physician. The size, weight, state of uremia, cardiac status, and general physical condition of the patient must be carefully evaluated by the prescribing physician before each treatment.
If patients suffer from acute kidney injury and / or volume overload, the Prismaflex ST set is indicated for continuous renal replacement therapies (CRRT), in modalities such as:
Slow Continuous UltraFiltration (SCUF)
Continuous Veno-Venous Hemofiltration (CVVH)
Continuous Veno-Venous HemoDialysis (CVVHD)
Continuous Veno-Venous HemoDiaFiltration (CVVHDF) to perform fluid management and reduction of uremic toxins.
The Prismaflex ST100 set and ST150 are indicated for use in patients with a body weight equal or greater than 30kg (66lb) and Prismaflex ST60 set is indicated to patients with a body weight greater than 11kg (24lb).
_____
INTENDED USE
The PrisMax control unit is intended for:
- Continuous Renal Replacement Therapy (CRRT) for patients weighing 20 kg or more with acute renal failure and/or fluid overload.
- Therapeutic Plasma Exchange (TPE) therapy for patients weighing 20 kg or more with diseases where removal of plasma components is indicated.
All treatments administered via the PrisMax control unit must be prescribed by a physician.
The Prismaflex control unit is intended for:
Continuous Renal Replacement Therapy (CRRT) for patients weighing 20 kilograms or more with acute renal failure and/or fluid overload.
Therapeutic Plasma Exchange (TPE) therapy for patients weighing 20 kilograms or more with diseases where removal of plasma components is indicated.
Continuous Renal Replacement Therapy (CRRT) in conjunction with the MARS system to conduct MARS treatments for patients weighing 20 kilograms or more.
All treatments administered via the Prismaflex control unit must be prescribed by a physician.
_____
INDICATION
MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins.
MARS is not indicated for the treatment of chronic liver disease conditions or as a bridge to liver transplant. Safety and efficacy has not been demonstrated for those indications in controlled, randomized clinical trials.
The effectiveness of the MARS device in patients that are sedated could not be established in clinical studies and therefore cannot be predicted in sedated patients.
Rx Only. For safe and proper use of the products mentioned herein, please refer to the appropriate Operator’s Manual or Instructions for Use.
Vantive, AN69, MARS, Prismaflex and Prismax are trademarks of Vantive Health LLC or its affiliates.