Oxiris Set
The Oxiris Set device is authorized under EUA200164 (Emergency Use Authorization) to treat patients 18 years of age or older with confirmed Coronavirus Disease 2019 (COVID-19) infection admitted to the intensive care unit (ICU) with confirmed or imminent respiratory failure in need of blood purification, including use in continuous renal replacement therapy. This device has neither been cleared or approved for the indication to treat patients with COVID-19 infection. The device is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of the Oxiris Set under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
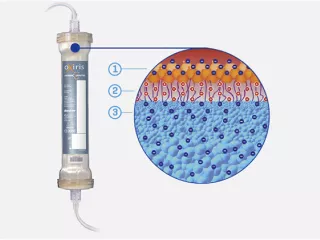
The Oxiris Set is only filterset available in the US that performs multiple blood purification therapies simultaneously, including continuous renal replacement therapy (CRRT) and the removal of cytokines and inflammatory mediators from the blood.
Clinical considerations
With or without CRRT, the Oxiris blood purification device is designed to remove inflammatory mediators in the treatment of COVID-19 patients.
Important Safety Information
Intended Use for Patients with COVID-19
The Oxiris Set is indicated for use only with the Prismaflex control unit or with the PrisMax control unit.
It is intended to treat patients 18 years of age or older with confirmed COVID-19 admitted to the ICU with confirmed or imminent respiratory failure in need of blood purification, including use in continuous renal replacement therapy, to reduce pro-inflammatory cytokine levels, who have any one of the following conditions:
- Early acute lung injury (ALI)/ early acute respiratory distress syndrome (ARDS); or
- Severe disease, defined as: dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates >50% within 24 to 48 hours; or
- Life-threatening disease, defined as: respiratory failure, septic shock, and/or multiple organ dysfunction or failure
The use of Oxiris is contraindicated (as mentioned in the IFU) when:
- Patients present a known allergy to heparin or have type II thrombocytopenia caused by heparin (HIT Syndrome type II)
- A drug used simultaneously with Oxiris is contraindicated per its Instructions for use
Relative contraindications (individual risk/benefit to be determined by treating physician) for the use of Oxiris include:
- The inability to establish vascular access to safely perform CRRT / hemoperfusion (SCUF; CVVH; CVVHD; CVVHDF)
- Severe hemodynamic instability
- Known hypersensitivity to any component of the Oxiris Set
This set is intended for use in the following veno-venous therapies: SCUF; CVVH; CVVHD; CVVHDF.
All treatments administered with the Oxiris Set must be prescribed by a physician. The size, weight, state of uremia, cardiac status, and general physical condition of the patient must be carefully evaluated by the prescribing physician before each treatment.
RX Only. For safe and proper use of the products mentioned herein, please refer to the Operator's Manual or Instructions for Use.
Vantive and Oxiris are trademarks of Vantive Health LLC or its affiliates.
References
-
Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(Suppl 3):1-14.
-
Thomas M, Moriyama K, Ledebo I. AN69: Evolution of the world's first high permeability membrane. Contrib Nephrol. 2011;173:119-129.
-
Harrori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Ren Replace Ther. 2016;2:41.