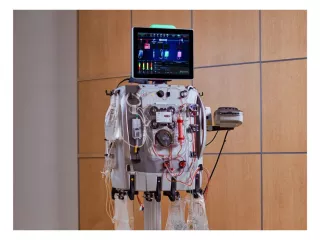
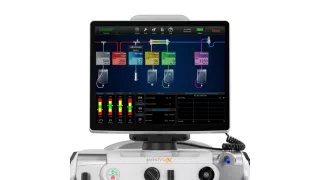
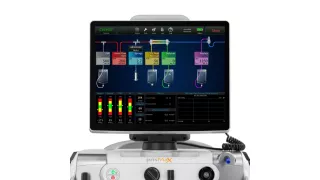
PrisMax Critical Care System
An advanced extracorporeal therapy system co-created with 650+ HCPs
We asked more than 650 ICU healthcare practitioners, from over 50 ICUs worldwide, what it would take to solve their greatest challenges in treating critically ill patients. The PrisMax System was designed to help meet these needs, building upon our Prismaflex technology and more than 30 years of experience in advancing critical care delivery.
Compatible Products
CRRT Solutions
Organ Support Filtersets
Digital Solutions
Unlock the potential of the ICU
The PrisMax System can help improve treatment accuracy, increase workflow efficiency, and simplify therapy setup and delivery.
Our assessment of the PrisMax System across seven ICUs in six countries concluded that the device features several important enhancements that contribute to safety, efficiency and user friendliness. The launch of PrisMax is a big step forward for clinicians’ abilities to treat patients in the ICU more effectively.1
Marcus Broman, MD
Department of Perioperative and Intensive Care of Skåne University Hospital in Lund, Sweden, and lead author of a publication about these results.
Important Safety Information
INTENDED USE
The Prismaflex and PrisMax Systems are intended for:
- Continuous Renal Replacement Therapy (CRRT) for patients weighing 20 kilograms or more with acute renal failure and/or fluid overload.
- Therapeutic Plasma Exchange (TPE) therapy for patients weighing 20 kilograms or more with diseases where fluid removal of plasma components is indicated.
The TherMax Blood Warmer Accessory is indicated for warming returning blood flow.
Rx Only. For safe and proper use of the products mentioned herein, please refer to the appropriate Operator’s Manual or Instructions for Use.
Vantive, Prismaflex, PrisMax and TherMax are trademarks of Vantive Health LLC or its affiliates.
References
-
Broman M, Bell M, Joannes-Boyau O, Ronco C. The novel PrisMax continuous renal replacement therapy system in a multinational, multicentre pilot setting. Blood Purif. 2018;46(3):220-227.